
Chlorine is a fundamental input to modern industry, yet most of today's supply still relies on energy-intensive electrolysis. In order to reduce energy consumption, researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) and the Technical Institute of Physics and Chemistry, both affiliated with the Chinese Academy of Sciences (CAS), have developed an alternative approach to producing chlorine—by harnessing the osmotic energy inherently stored in chloride-rich brines.
In a study recently published in Nature Communications, the researchers detail a modular system that integrates hydrochloric acid recovery with chlorine and hydrogen generation—eliminating the need for external electrical input during the electrochemical stage.
The concept builds on diffusion-cell technology already employed industrially for waste acid recycling. Within each power-generation unit, a chloride-rich waste acid feed flows toward a proton-selective membrane. As protons diffuse across the membrane, a permeate stream forms on the opposite side, where purified acid is collected. To maintain charge balance, electrons flow through an external circuit, generating electrical output that powers downstream reactions.
A critical enabling component is a sulfonated covalent-organic framework (COF) membrane. By facilitating rapid proton transport while blocking multivalent metal ions, the membrane ensures the recovered acid remains cleaner and mitigates performance losses associated with metal-induced side reactions.
To sustain operation and regulate chloride transport, the system utilizes a reversible Ag/AgCl redox couple with periodic electrode switching. In stacked configurations with graphite anodes and platinum cathodes as terminal electrodes, the system drives chlorine and hydrogen evolution in separate compartments.
Tests using simulated waste acid showed that the integrated device achieved chlorine and hydrogen production rates of approximately 150 L m-2 h-1 and operated stably for at least seven days. The team also demonstrated feasibility with simulated desalination wastewater and seawater, although output levels were lower.
"Waste brines are often viewed as liabilities," said ZHU Chenguang at QIBEBT, first author of the study. "Here, we demonstrate they can serve as both an energy source and a feedstock—recovering acid while producing two high-value gases."
"What distinguishes this system is its compatibility with existing industrial processes, such as diffusion dialysis, which is already used for acid recovery across multiple industries," added Prof. GAO Jun at QIBEBT, corresponding author of the study. "This enhances the technology's scalability and ease of integration into current infrastructure."
The researchers note the same osmotic-electrochemical coupling principle could be extended beyond chlorine production to other brine-based reactions—including ammonia production from nitrate-containing brines—thereby unlocking new possibilities for low-carbon chemical manufacturing.
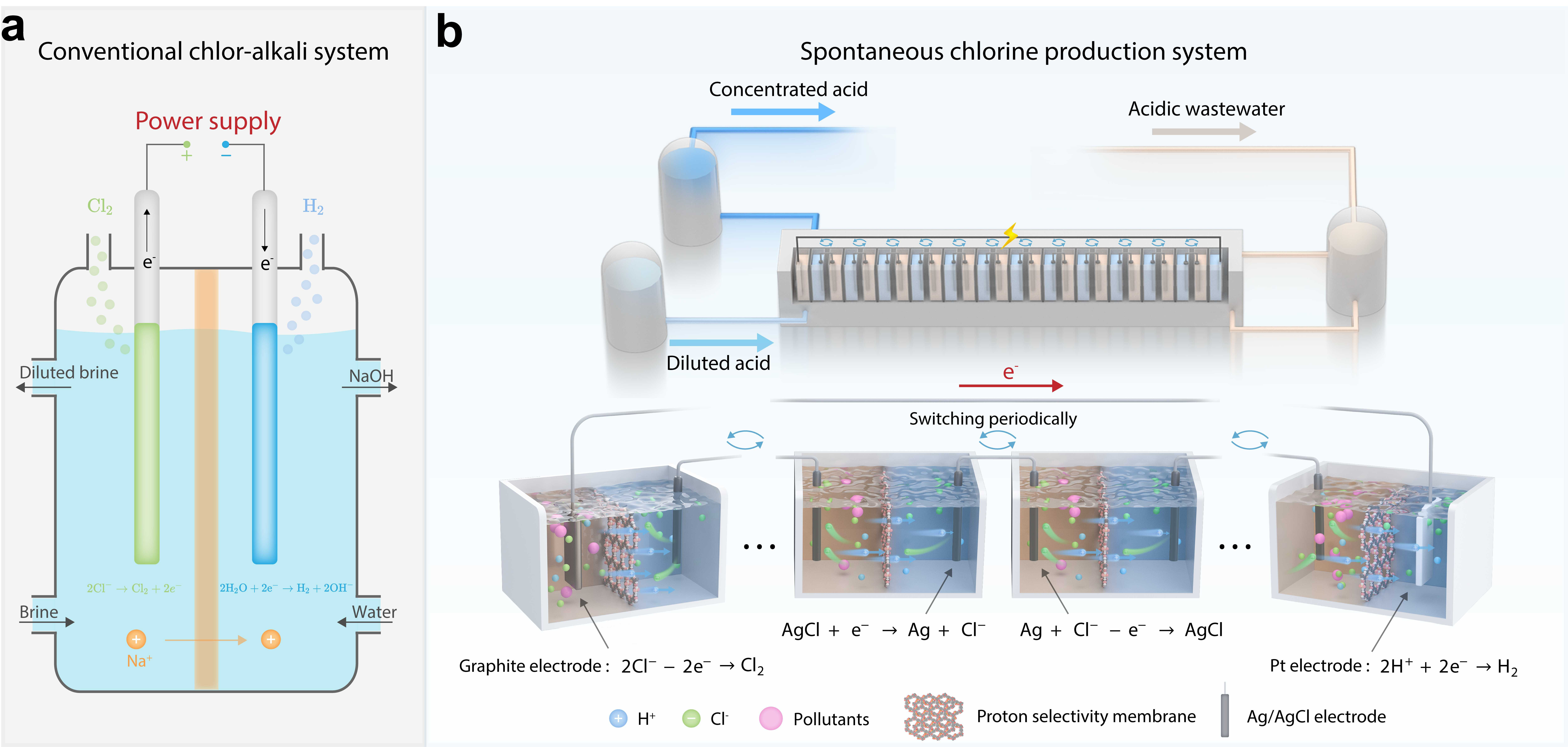
Schematic illustration of conventional chlor-alkali system and spontaneous chlorine production system. (Image by QIBEBT)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

